|
Madison Farrish 9/22/06 chemsitry p. 6 [4/4] Answer the following fully and completely using nouns, verbs, and ly-words. [9/10] 1. Complete the table using your Periodic APP or the 50th
Year of ChemEd table.
2. Determine the density of the block that Mr. Young assigns you. V= 7.6*12.7*2=193.04 cm^3 M= 144 g 144/193.04= .74 g/cm^3 I had the Blank Index Cards, and the density of them is .74 g/cm^3. I got the density by dividing the mass—144g--- by the volume---193.04cm^3. [14/15] 3. If the Lead Brick was really Gold – How heavy would it be? Give the answer in 4 different units. 11.34 g/cm^3 is the density of lead. The density of gold is 19.3. Volume of lead is 395.92cm^3 19.3* 395.92^3= 1197787100 gà 2.640669E6 lbmà1.197787E6 kgà 8.207449E4 slug à 1.320334E3 ton I got these answers by using the unit converter on my calculator. [5/25] [wrong brick] 4. We are interested in the amount of lead in 55 cans of paint. There should be 0.047 centigrams of lead in each can. Each can has a mass of 0.023 slugs. If we perform a T-Test on the data collected from these cans, at what Level of Significance would you test? Why? I think it would have a level of significance .04, because the amount of lead in the paint is very important, because lead can be very deadly or toxic. For example, if I painted my fence and my dog came up and licked it a lot, it would die if the lead in the paint wasn’t equal to the amount it needed to be. That’s how toxic lead is. [15/15] 5. Determine the ppb of lead for the paint in question 4. First you convert .047 centigrams into grams and .023 slugs into grams. So now I have .00047 grams from my centigrams, and 3.356598E2 g from my slugs. So know you dive .00047g by 335.6598 and times it by a billion. 1.400227254E-6 * 1000000000= 1400.23. The ppb for lead in question 4 is 1400.23 [15/15] 6. Convert these and fill the table I used the unit converter in my calculator to figure this out.
7. ROSES and BEARS: Use screen shots of each to explain the
relationship between Roses/Petals/Roots and Bears/Fish. [Just compare one
pair.] [12/20] 8. Using the LEAD list, perform a T-Test using the LOS from question 4. Report the results and your conclusion(s). - After I did the T-test I compared the answer I got p= .0278 to the level of significance I chose, .04. This means that I would accept the amount of lead in each paint-can, because .0278 is lower than the level of significance I chose. [20/20] sweet!] |
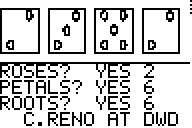
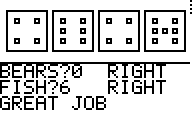 I think both
roses and fish are alike. To find roses, you look at the middle of the dice to
see if there is a middle dot, and if there is one then that dice is a rose. You
also look to the middle of the dice when looking for fish. If there is a dot
then you look to the right and left of the dot and count how many dots
aren’t there.
I think both
roses and fish are alike. To find roses, you look at the middle of the dice to
see if there is a middle dot, and if there is one then that dice is a rose. You
also look to the middle of the dice when looking for fish. If there is a dot
then you look to the right and left of the dot and count how many dots
aren’t there.